In recent years, significant progress has been made globally in disease prevention and treatment, including HPV vaccines, gene therapy, and insulin biosynthesis, effectively extending life expectancy. These achievements cannot be achieved without an innovation ecosystem built on the patent system. The monopoly gains within a certain period conferred by the patent system are indispensable to support the enormous R&D investment in the pharmaceutical industry. However, high prices for patented medicines have also sparked widespread criticism of the patent system. Large numbers of the world’s population are still nagged by insufficient availability of medicines or unaffordable health care costs. This problem is especially pronounced in developing countries.
When patents’ exclusive rights contradict public health issues, how to balance the patentee and the public interest has become a widely concerned policy issue. The importance of this issue has been further highlighted in the context of the COVID-19 pandemic. We must revisit the relevant conclusions from the TRIPS Agreement and the Doha Declaration on Public Health, summarize, and learn our lesson. We also need to explore a patent system in harmony with public health interests and provide policy and legal infrastructure to expand new technologies’ development and production potential. Discussions on patents and public health also help us rethink innovation and IP management from a more open and inclusive perspective.
In this context, on July 5, 2022, the United Nations (UN) World Intellectual Property Organization (WIPO) and Tongji University jointly invited Professor William Fisher from Harvard University in the US, Professor Stefan Wagner from the European School of Management and Technology in Germany (ESMT Berlin), and Associate Professor Sven Bostyn from the University of Copenhagen in Denmark to give three academic reports focused on patents and public health issues. More than 200 people, including teachers and students from the SICIP of Tongji University, and other member units of WIPO International Alliance for Intellectual Property Education (WINIPe), attended the seminar online. The report meeting also attracted enthusiastic participation and in-depth discussions from other domestic universities and industry circles.
Prof. Fisher’s Lecture
The lecture by Professor William Fisher of Harvard Law School was presided over by Associate Professor YU Xinmiao, Executive Vice Dean of the SICIP. Professor Fisher gave the speech titled “The Global Health Crisis: A Bird’s-Eye View of Problems and Solutions.” Based on global mortality and morbidity data for various diseases in 2019, he pointed out that infectious and parasitic diseases had become the world’s two leading causes of death, which were major threats to life expectancy in low-income countries. Based on his historical experience in preventing and controlling infectious diseases in the United States, he noted that the public health system, vaccines, and drugs are critical factors in reducing the fatality rate of contagious diseases. In contrast, limited access to drugs and vaccines, defective and counterfeit drugs, and little new drug innovation are the core factors limiting the ability of low-income countries to prevent and control infectious diseases. Professor Fisher suggested reducing the imbalance of medical resources between low-income and high-income countries and reducing unnecessary deaths in low-income countries. In the commentary and Q&A session after the lecture, Professor LI Mingde of the Chinese Academy of Social Sciences pointed out that drug innovation is crucial to solve the health crisis, combining China’s experience in solving food problems through agricultural technology innovation. Professor SONG Xiaoting of the SICIP introduced China’s experience in balancing drug patent protection and public health interests from the perspectives of the expansion of patentable biomedical objects, compulsory licenses, and open licenses. Scholars at the conference had lively discussions on issues such as the right to life, compulsory licenses, open licenses, patent monopoly rights, and vaccine patents.
Prof. Bostyn’s Lecture
Professor SONG Xiaoting, Secretary of the Party Committee of the SICIP, presided over the academic lecture of Associate Professor Sven Bostyn of the Law School of the University of Copenhagen, Denmark. Associate Professor Bostyn gave the academic report titled “The challenges in marrying access to health and exclusive rights to medicinal products.” Starting from the supply and demand structure of the pharmaceutical market, he proposed that pricing and compensation rules will significantly affect the competition among drugs. Bringing new drugs to market by pharmaceutical companies required overcoming multiple hurdles with high risk and uncertainty. Professor Bostyn further analyzed the push and pull incentives of the pharmaceutical industry and analyzed the conflict between public health and drug patent rights in detail from three areas: drug reuse, rare drugs, and superimposed indications. He also suggested building a drug patent system that could make innovation incentives and public health interests compatible. In the commentary and Q&A session after the lecture, Professor Joseph Straus, former director of the Max Planck Institute for Innovation and Competition in Germany, commented on the solution proposed by Associate Professor Bostyn, pointing out that the corresponding solution needs to be based on empirical data. Lawmakers should be cautious without corresponding data. From the comparative law perspective, Associate Professor PENG Zhe of Shandong University Law School introduced the intervening rights in US law, similar to the compulsory license system. According to the US Congress report, he mentioned that though there were a small number of additional compulsory licenses for US patents, there were no cases of compulsory licenses in the US. Scholars at the meeting had heated discussions on issues such as the Bayh-Dole Act, compulsory licenses, drug patent linkage, compensation for drug patent terms, and drug data exclusivity.
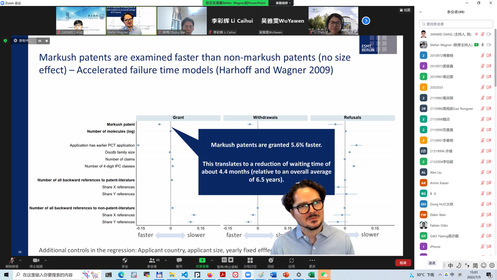
Prof. Wagner’s Lecture
Professor Stefan Wagner from ESMT Berlin presented his newly published paper “Mapping Markush” in Research Policy, which explored the impact of Markush patents on patent examination and pharmaceutical innovation. The Markush structure is a special chemical molecular structure, and patent claims written in the Markush style could protect a class of chemicals with similar structures. There are many disputes between academia and industry on the examination burden and patent barriers of Markush patents. However, empirical research has not tested these disputes for a long time. Based on a large number of medical patent data, Prof. Wagner empirically proved for the first time that, contrary to popular belief, the Markush patent did not lead to an extension of the patent examination period. Yet it did make it easier to form patent barriers and reduce the authorization rate of subsequent invention patent applications. IP directors and drug R&D project directors from well-known pharmaceutical companies, and academic researchers had a lively discussion around Professor Wagner’s report. Experts in the industry generally believed that the current protection of Markush patents in our country is more reasonable in balancing the interests of innovators and the public interests. Although the Markush patent formed a barrier to micro-pharmaceutical innovation by fine-tuning the molecular structure, it could encourage pharmaceutical companies to develop more technical directions for drug research and development, objectively increasing the types of new drugs available to the public. Associate Professor HUO Dong from Harbin Institute of Technology (Shenzhen), Assistant Professor CHEN Li from the University of Gothenburg, Sweden, Professor REN Shengce from the SICIP, Assistant Professor LIU Xia from the SICIP, etc. had in-depth discussions with Professor Wagner and industry experts on issues such as the package licensing of Markush patents, decision-making mechanism for corporate drug R&D and patent layout strategies, and Markush patent review cycle. Professor Wagner’s seminar was hosted by Associate Professor Jianwei Dang of the SICIP.






 0086-021-65983113
0086-021-65983113  sicip_intoff@tongji.edu.cn
sicip_intoff@tongji.edu.cn